Antibody Therapy R&D trends and breakthrough innovations
This report provides a review of the latest R&D trends in antibody therapies, surfacing the most promising breakthroughs being developed by teams in research institutes and biotech companies around the world.
The trends and breakthroughs in the report are identified by analyzing the engagement of External Innovation and R&D teams using our online partnering platform (join here) to identify their next scientific partners.
These insights should provide scientific decision-makers with a roadmap of high-impact opportunities in an evolving and competitive landscape, pinpointing emerging technologies and potential partners to accelerate the development of new antibody therapies.

Download the report
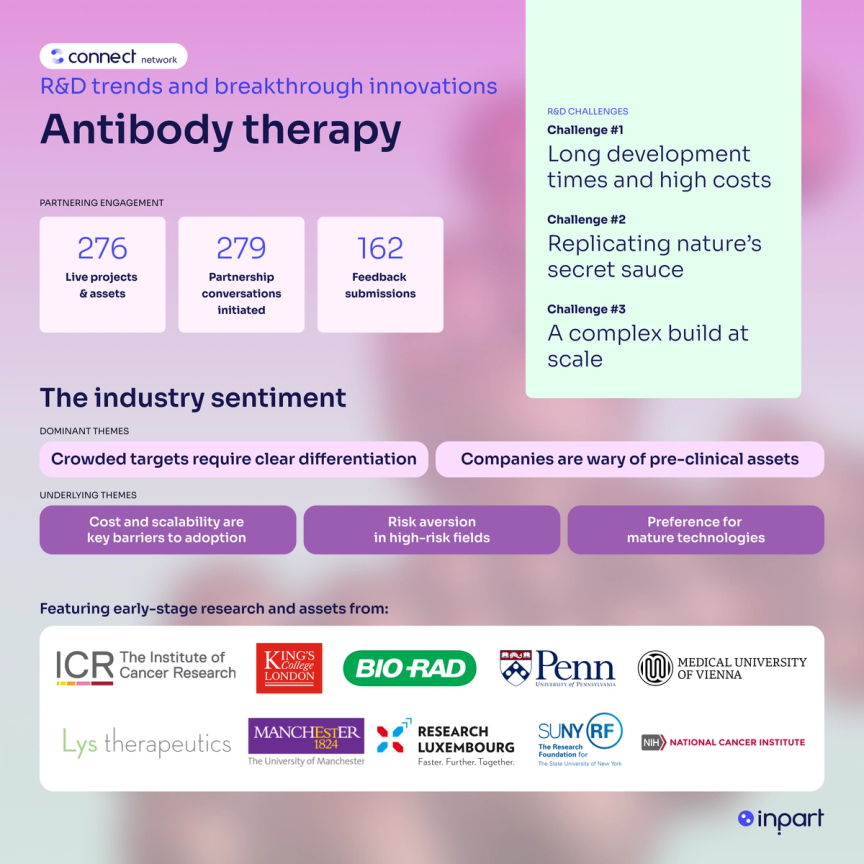
Key R&D challenges for antibody therapy
Antibody therapies are a cornerstone of modern medicine. Their use has evolved since the first monoclonal antibody developed by Ortho Pharma/J&J was approved by the FDA in 1986. Harnessing these Y-shaped proteins, produced naturally by the immune system, has led to the development of breakthrough therapies for cancer, autoimmune disorders, and infectious diseases.
The high specificity of each antibody means they can target diseased cells without harming healthy ones, minimizing side effects, offering opportunities where conventional therapies fail. Their effectiveness has led to antibody therapies becoming some of the best-selling drugs on the market, a trend which is projected to continue.
Antibody therapies are also driving advancements in personalized, tailored medicine. They have enhanced vaccine development, diagnostic tools, and immunotherapy, transforming disease management across many medical fields. Their use is continually expanding the possibilities for treating complex and previously untreatable conditions and disease.
R&D challenge #1
Long development times and high costs
Antibodies can take decades to develop as they require extensive preclinical testing, clinical trials, and regulatory approval. This is challenging for companies investing in antibody therapy R&D because it increases financial risks, as well as delaying access to life-saving treatments. High costs are often passed on to patients, making treatments less affordable and accessible, particularly in low-income economies.
R&D challenge #2
Replicating nature’s secret sauce
Specificity, precision and targeted delivery are difficult to design in antibodies created outside the human body. Even slightly off-target interactions can have significant side effects or reduce effectiveness. The delivery of antibodies to the sites of disease can often be ineffective, with one study reporting only 2% of an antibody-drug conjugate dosage reaching the tumor site. With some diseases and conditions, resistance and tolerance can arise to antibody treatments, particularly with cancer and chronic diseases.
R&D challenge #3
A complex build at scale
Manufacturing antibody therapies at scale is challenging, not least because antibodies are large, delicate and intricate proteins requiring specialized processes and facilities. Living cells are required to manufacture antibodies through mammalian or bacterial systems under precise, highly-controlled conditions. Scaling up from success in a small labs to global production further complicates things; small changes in the environment can amplify through production, risking efficacy and safety.
Antibody Therapy projects and assets
Review a sample of the six highest performing antibody therapy projects and assets hosted on our online partnering platform.
To read the full summary article for each technology, asset or project, and to connect with the team developing them, you will need to join the platform.

1. A first-in-class mAb treating neurological diseases and restoring the blood-brain-barrier
In neurological diseases such as stroke, multiple sclerosis, and Parkinson’s, dysfunction of the blood-brain barrier allows inflammatory cells into the brain, causing neuroinflammation and neuronal death. However, current treatments struggle to address the root cause and target inflammation at its source.
Researchers at Lys Therapeutics have developed a monoclonal antibody that preserves and restores the blood-brain barrier (BBB) by blocking its degradation without crossing the barrier itself. Through their mAb, tight junctions are reestablished, endothelial cells return to a healthy state, and the BBB is restored. The team are seeking partners for co-development, licensing and investment.
2. Diverse antibodies enhancing the immune response to tumors
One method of immunotherapy involves targeting immune checkpoints that suppress Tcell responses. TIGIT, a receptor found in tumor-infiltrating lymphocytes, plays a key role in this process, withthe ligan CD155 on tumor cells interacting with it to suppress the immune system. Addressing this mechanism is critical for improving outcomes in cancer patients.
Using Bio-Rad's Pioneer Antibody Discovery Platform, Bio-Rad researchers have developed ten diverse antibodies targeting TIGIT. Their antibodies block the TIGIT/CD155 interaction, restoring immune function and enhancing anti-cancer responses. Preclinical tests have shown promising results, with the team now seeking partners and licensing.
3. A first-in-class mAb treating immune-resistant cancers and immunological disorders
Immune checkpoint inhibitors have transformed cancer treatment, but some tumors remainresistant. This is often due to immune-suppressive factors including TLT-1, a protein secreted by activated platelets. As TLT-1 is linked to poor survival rates in cancer patients, targeting this mechanism could improve the immune response to resistant tumors.
At Ascendo Biotechnology, researchers have developed a monoclonal antibody known as ASD141 which works to block TLT-1 and disrupt the tumor's suppressive environment. In preclinical studies, the Ascendo researchers have demonstrated that ASD141, combined with existing immune therapies, enhanced survival in animal models and inhibited tumor growth. Their innovation has the potential to expand treatment options for solid tumor cancers, and the team are seeking partners for investment and co-development.
4. Reducing inflammation caused by T cell infiltration in autoimmune diseases
Autoimmune diseases, affecting over 24 million people in the USA, lead the immune system to attack healthy tissues, causing significant damage and chronic inflammation. Conditions such as psoriasis, multiple sclerosis, and inflammatory bowel disease are driven by Tcell infiltration into inflamed tissues. This process is, in part, mediated by the chemokine CCL21, providing a key target for new treatments aiming to limit disease progression.
Researchers at UT Health San Antonio have developed a monoclonal antibody that neutralizes CCL21, blocking Tcell migration and reducing tissue damage in autoimmune conditions, with less broad immunosuppression. This treatment would be the first of its kind; there are currently no FDA approved CCL21 Mabs. The researchers are seeking licensing and collaboration partners.
5. An antibody discovery platform targeting complex membrane proteins
Targeting complex membrane proteins such as ion channels and G protein-coupled receptors is a focus for R&D in many diseases. However, due to the challenges of identifying accessible binding sites for antibodies on these proteins, the development of effective treatments has been limited.
AbiProt®, a platform developed by researchers at Oblique Therapeutics, addresses this issue by using microfluidics and proteases to map accessible antibody-binding sites on native-state cell membrane proteins. It has successfully generated functional antibodies for the TRPV1 ion channel, validated in non-human primates. Their platform holds potential for developing new therapies for previously hard-to-target proteins, and the researchers are seeking licensing and partnerships.
6. A targeted immunotherapy for ovarian cancer
Epithelial ovarian carcinoma (EOC) occurs primarily in post-menopausal women and is typically diagnosed at late stages, resulting in a high recurrence rate and a low five-year overall survival rate. The anti-Mullerian hormone receptor II (AMHR2) is expressed in most EOCs, providing a clear target for treatment as it is expressed in ovarian tissue but absent in post-menopausal ovaries.
At Cleveland Clinic, researchers have been working towards this, developing a monoclonal antibody against AMHR2 with demonstrable results in vivo. The antibody is humanized, high-affinity, and can be used on its own or in an antibody drug conjugate. The researchers behind the technology are seeking licensing and a partner for co-development.
Listen to an audio digest of the editorial and report introduction
Join a global partnering network with 17,000 scientists and industry decision-makers
We connect academic institutes, researchers, companies and investors in a first-of-its-kind online network, unlocking the transformative power of science through partnering